How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora
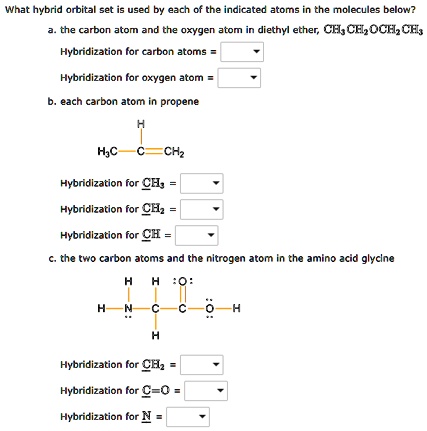
SOLVED: Text: What hybrid orbital set is used by each of the indicated atoms in the molecules below? a. the carbon atom and the oxygen atom in diethyl ether, CH3COCH2CH3 Hybridization for
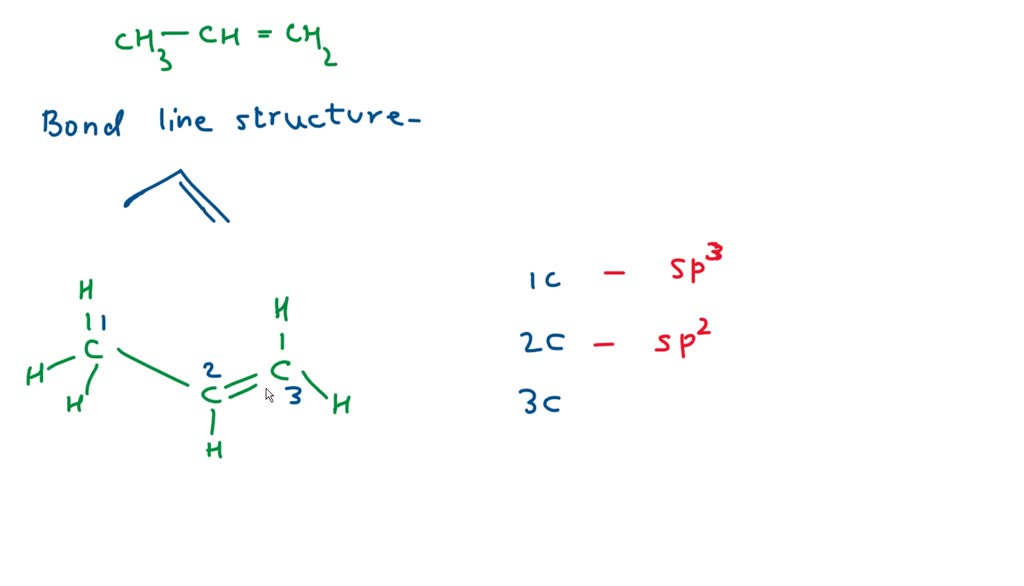
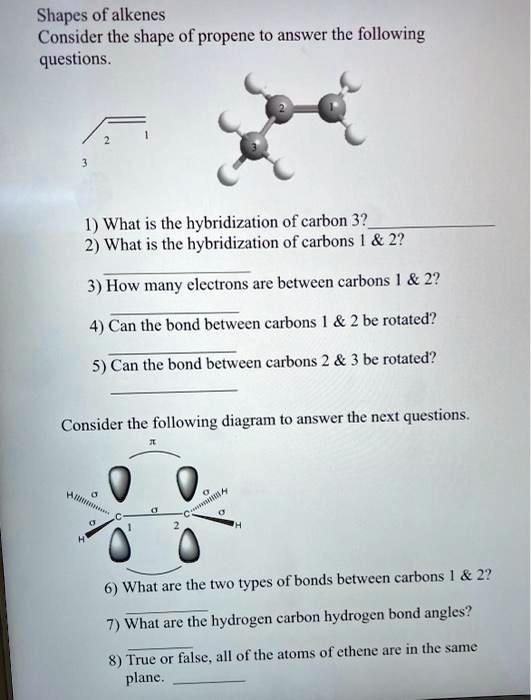
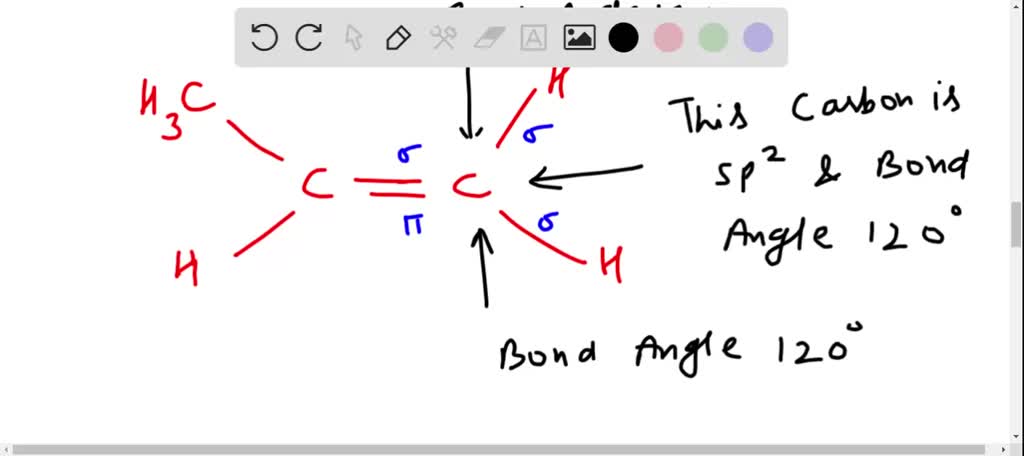
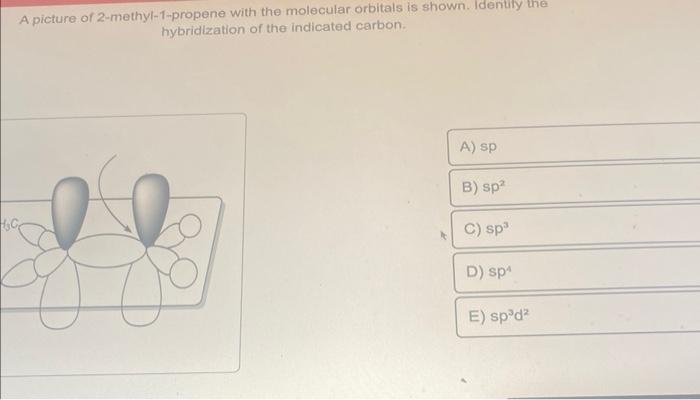
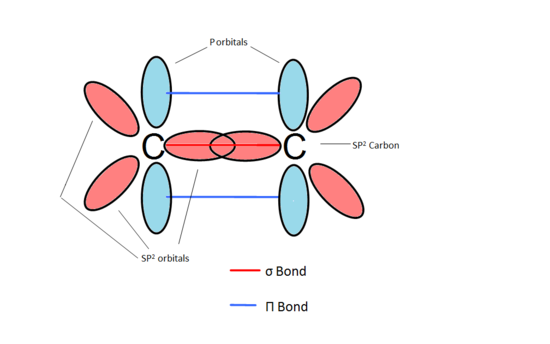
SOLVED: Draw a line-bond structure for propene, CH3CH=CH2. Indicate the hybridization of the orbitals on each carbon, and predict the value of each bond angle.
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora

Scheme 3. Conformations of propene in the s-p representation (3, 4)a nd... | Download Scientific Diagram
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...