
Orbital polarization and pd hybridization Ni L3-edge X-ray absorption... | Download Scientific Diagram
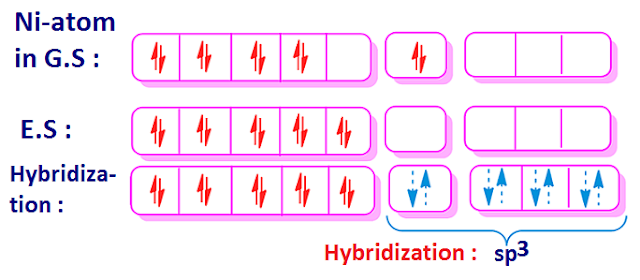
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Specifically, why is (Zn(CN)4)2- tetrahedral while (Ni(CN)4)2- is square planar, and why is (CuCl5)3- trigonal bipyramidal while (MnCl5)3- is square pyramidal? | Homework.Study.com
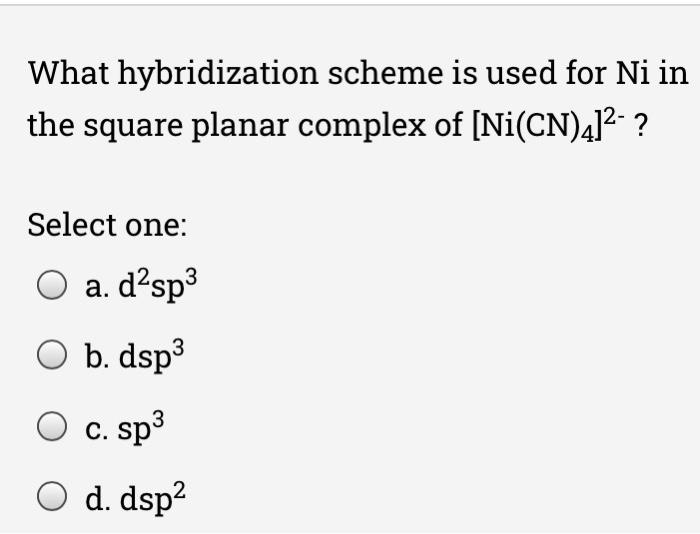
![Hybridization and geometry of [Ni(CN)_{4}]^{2-} are:sd^{3} and square planarsp^{2}d and tetrahedralsp^{3} and tetrahedraldsp^{2} and square planar Hybridization and geometry of [Ni(CN)_{4}]^{2-} are:sd^{3} and square planarsp^{2}d and tetrahedralsp^{3} and tetrahedraldsp^{2} and square planar](https://search-static.byjusweb.com/question-images/toppr_ext/questions/1453910_739662_ans_1b88a50e6b3142878d714df46d9472b7.png)
Hybridization and geometry of [Ni(CN)_{4}]^{2-} are:sd^{3} and square planarsp^{2}d and tetrahedralsp^{3} and tetrahedraldsp^{2} and square planar

Nd 5d–Ni 3d orbital hybridization and CDW in NdNiO2 and superconducting... | Download Scientific Diagram
How to find the hybridizationof[Ni(CN)4]2 n ntand the d orbital used in it. What is the hybridization of NO2?
![Explain hybridization, magnetic property and structure of [Ni(dmg)2]+2 - Chemistry - Coordination Compounds - 11977560 | Meritnation.com Explain hybridization, magnetic property and structure of [Ni(dmg)2]+2 - Chemistry - Coordination Compounds - 11977560 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_5a265991a58d6.jpg)
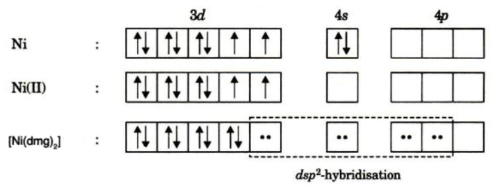
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:ffa0132b7d904ae4be8cc8b622fc0250.png)
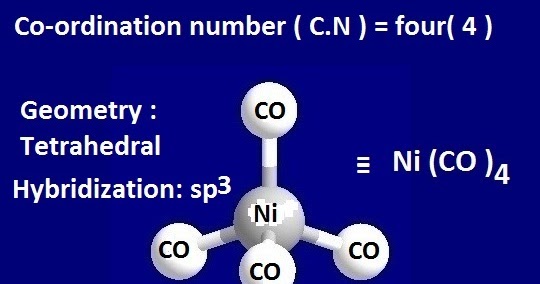

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)

![What is the hybridization of [Ni(co) 6] 4+? - Quora What is the hybridization of [Ni(co) 6] 4+? - Quora](https://qph.cf2.quoracdn.net/main-qimg-20bb619759136ec1b4419262dd5a28a0.webp)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
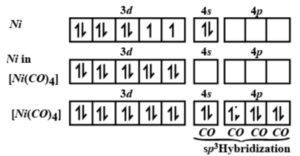
![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/mqdefault.jpg)