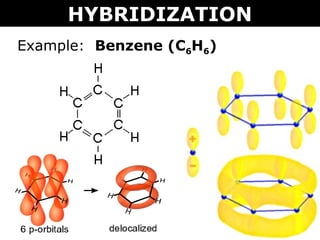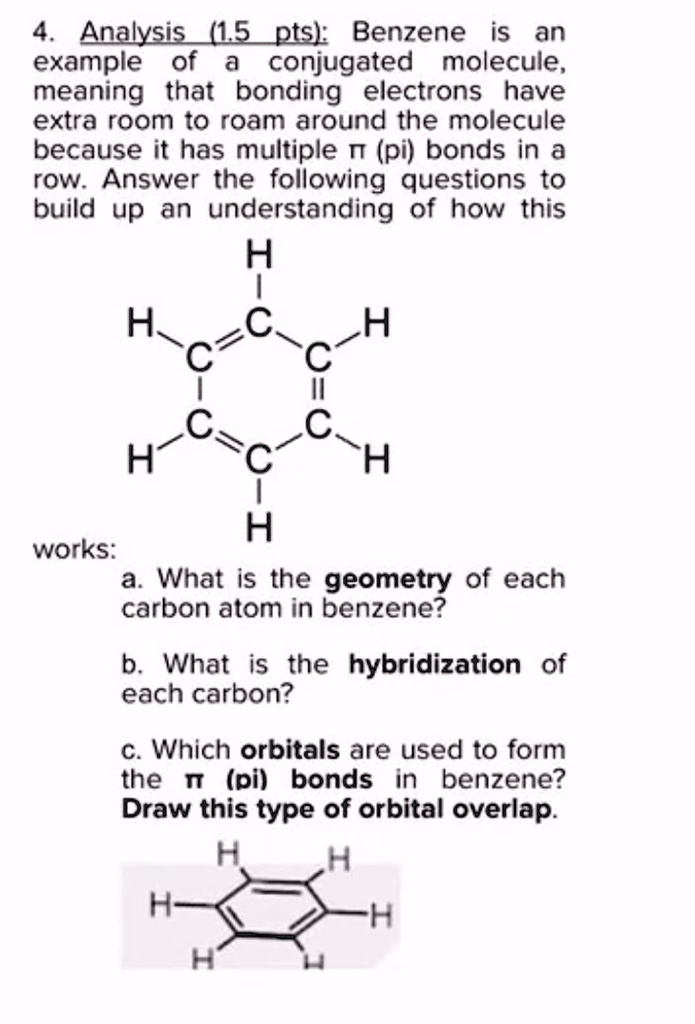
SOLVED: Benzene is an example of a conjugated molecule, meaning that bonding electrons have extra room to roam around the molecule because it has multiple π (pi) bonds. In order to understand
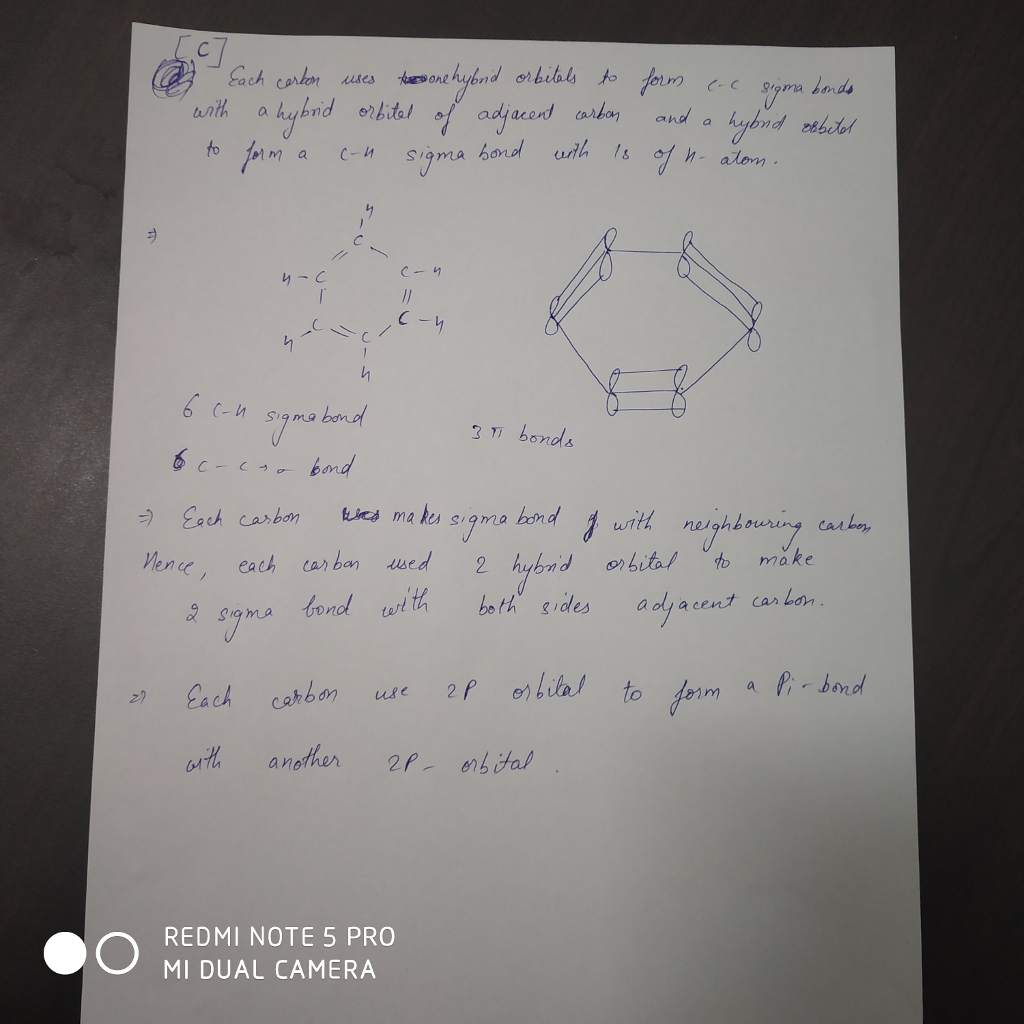
OneClass: What is the hybridization of the carbon atoms in benzene, C6H6? a. sp b. sp2 c. sp3 d. sp3d

What is the hybridization of each carbon atom in benzene? What shape do you expect benzene to have? | Homework.Study.com

Benzene Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram - Techiescientist
 \\[s{p^3} and {\\rm{ }} s{p^2}\\](C) \\[Both{\\rm{ }}\\,s{p^2}\\](D) \\[Both{\\rm{ }}\\,s{p^3}\\] What is the hybridization state of B and N in inorganic benzene respectively?(A) \\[s{p^2} and {\\rm{ }} s{p^3}\\](B) \\[s{p^3} and {\\rm{ }} s{p^2}\\](C) \\[Both{\\rm{ }}\\,s{p^2}\\](D) \\[Both{\\rm{ }}\\,s{p^3}\\]](https://www.vedantu.com/question-sets/9dddfcd2-409b-471f-9a63-d868bcb8b4ec222029276479968829.png)
What is the hybridization state of B and N in inorganic benzene respectively?(A) \\[s{p^2} and {\\rm{ }} s{p^3}\\](B) \\[s{p^3} and {\\rm{ }} s{p^2}\\](C) \\[Both{\\rm{ }}\\,s{p^2}\\](D) \\[Both{\\rm{ }}\\,s{p^3}\\]
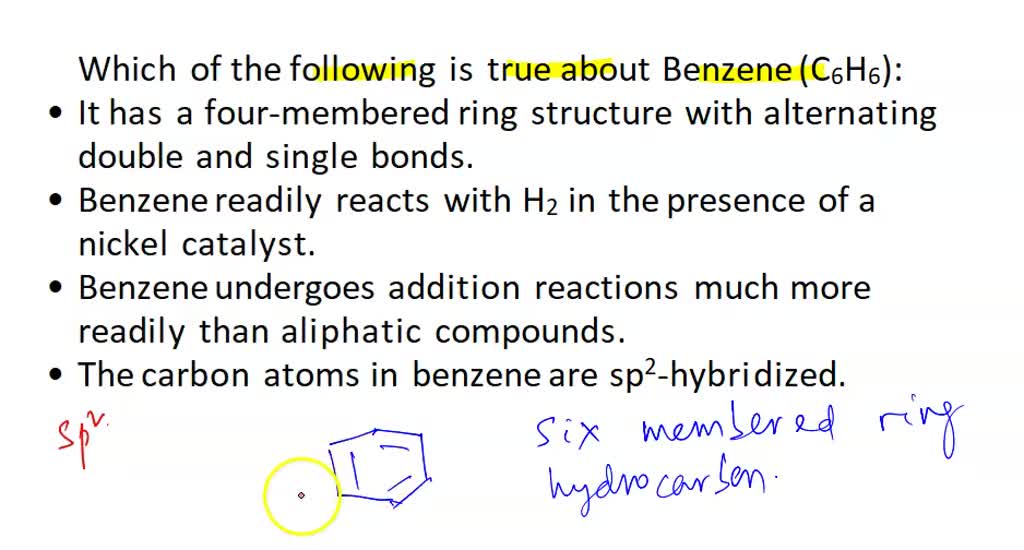
SOLVED: Which of the following is true about Benzene (C6H6)? It has a six-membered ring structure with alternating double and single bonds. Benzene readily reacts with H2 in the presence of a

C6H6 lewis structure, molecular geometry, bond angle, hybridization | Molecular geometry, Molecular, Molecular shapes


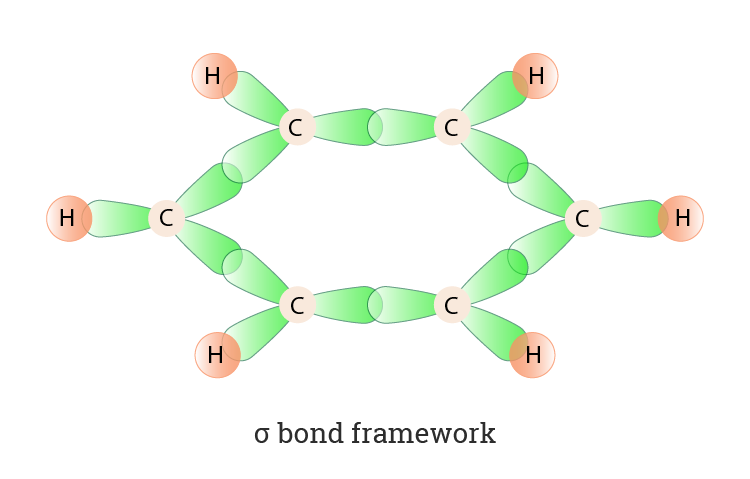
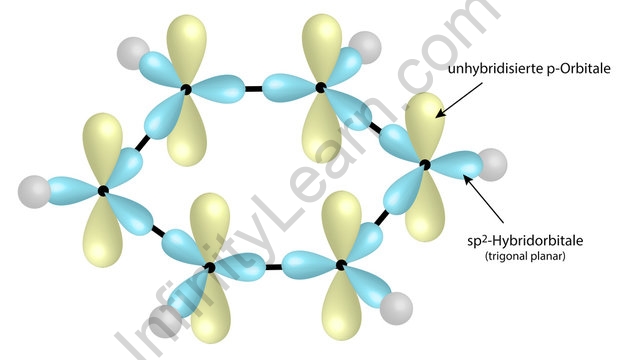
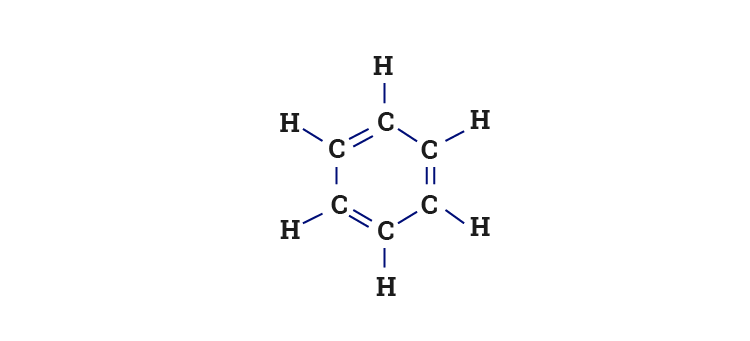
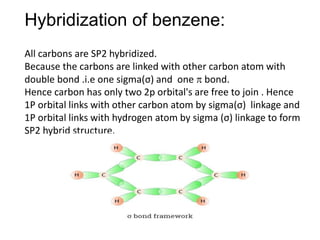


![The number of \\[s{p^3} - s\\] sigma bonds in benzene is:A.3B.6C.12D.none The number of \\[s{p^3} - s\\] sigma bonds in benzene is:A.3B.6C.12D.none](https://www.vedantu.com/question-sets/6c01f233-1562-4d6e-b183-b09dcf51bc951429559277162239695.png)