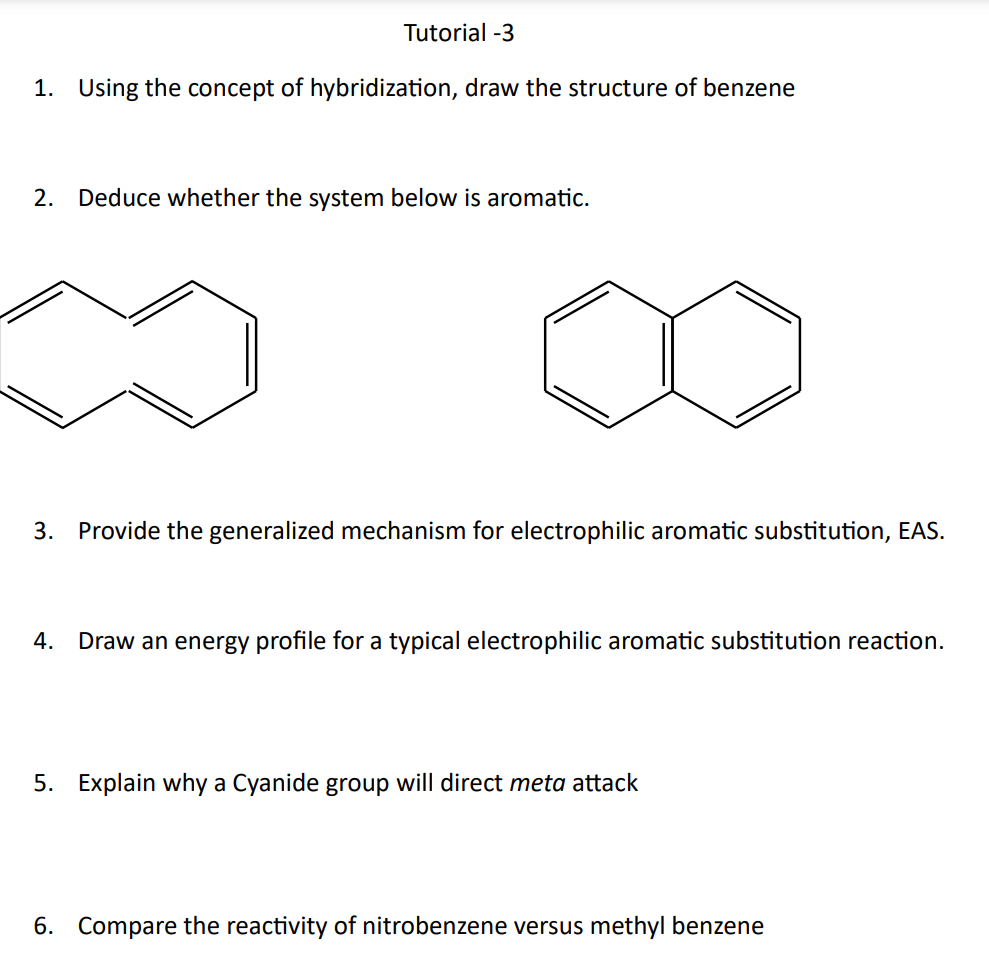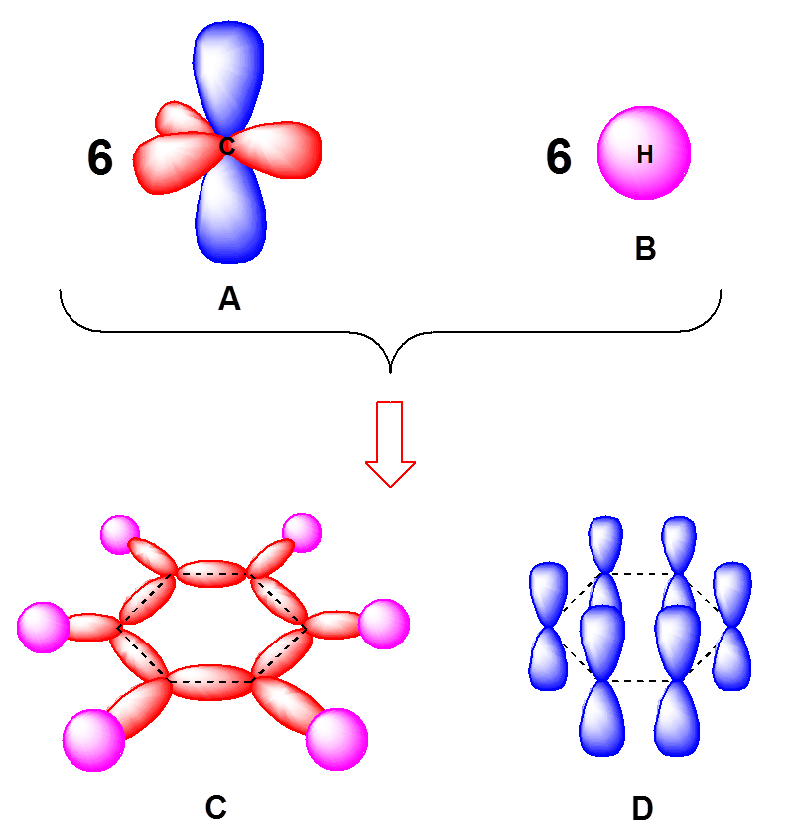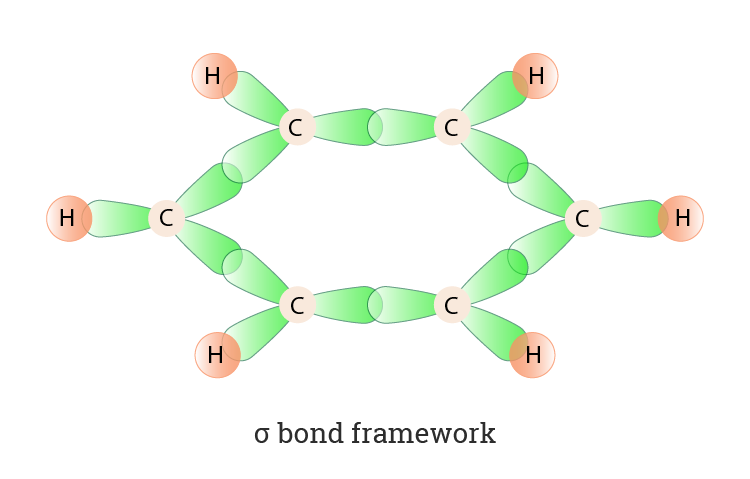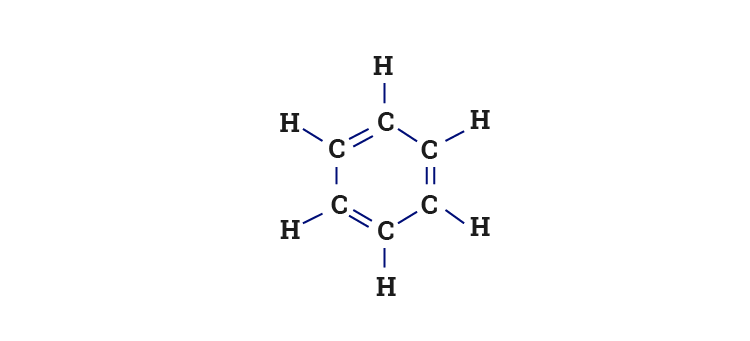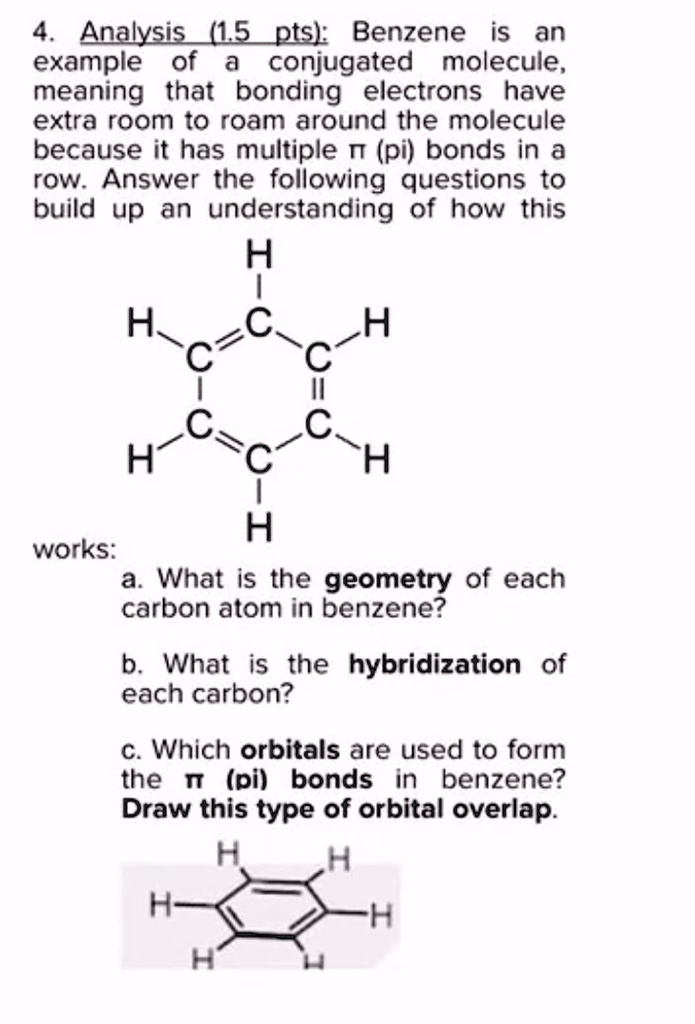
SOLVED: Benzene is an example of a conjugated molecule, meaning that bonding electrons have extra room to roam around the molecule because it has multiple π (pi) bonds. In order to understand
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora

SOLVED: 1. a) Label the hybridization of each carbon atom in benzene Next draw 3-D representation of benzene clearly showing all pi orbitals (including electrons) How does this demonstrate that the pi

Benzene Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram - Techiescientist

What is the hybridization of each carbon atom in benzene? What shape do you expect benzene to have? | Homework.Study.com

Benzene C₆H₆ : Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic
 \\[s{p^3} and {\\rm{ }} s{p^2}\\](C) \\[Both{\\rm{ }}\\,s{p^2}\\](D) \\[Both{\\rm{ }}\\,s{p^3}\\] What is the hybridization state of B and N in inorganic benzene respectively?(A) \\[s{p^2} and {\\rm{ }} s{p^3}\\](B) \\[s{p^3} and {\\rm{ }} s{p^2}\\](C) \\[Both{\\rm{ }}\\,s{p^2}\\](D) \\[Both{\\rm{ }}\\,s{p^3}\\]](https://www.vedantu.com/question-sets/9dddfcd2-409b-471f-9a63-d868bcb8b4ec222029276479968829.png)