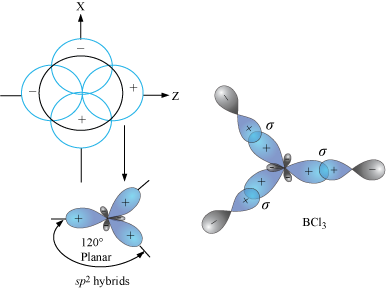
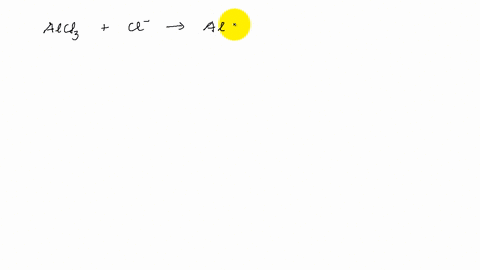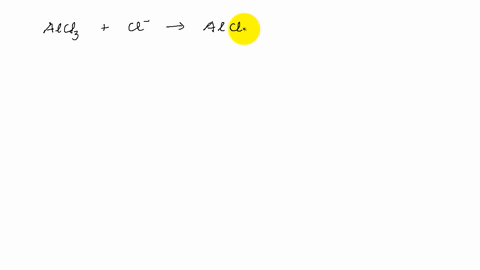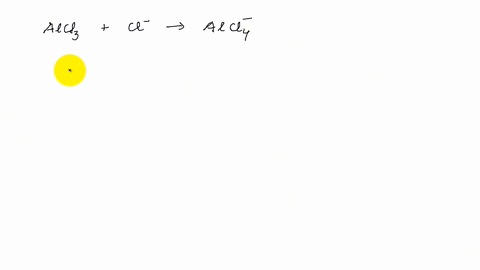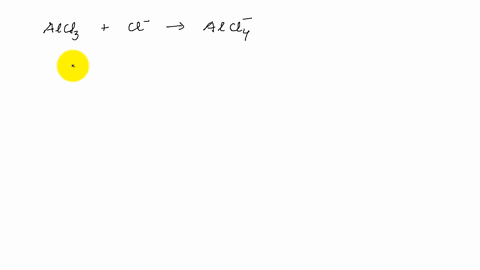AlCl3 Lewis Structure, Molecular Structure, Hybridization, Bond Angle, and Shape - Geometry of Molecules

The change in hybridization of aluminium when `Al_(2)Cl_(6)` decomposes in the gas phase is : - YouTube

There is no change in the hybridization of B and N atoms as a result of the following reaction: BF3 + NH3→F3B.NH3
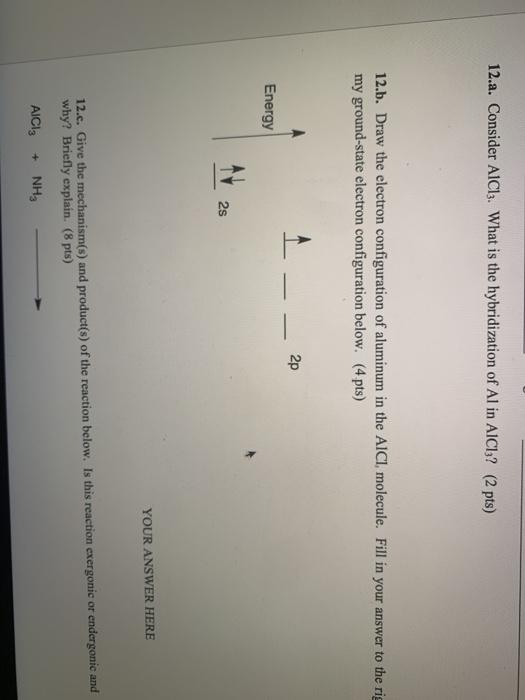
4. q hybridisation of Al in AlCl3 (monomeric form above 800C) and Al2Cl6(dimeric form below 400C) respectively are?

What is the molecular structure of the molecule AlCl3? What characteristic is notable about the structure that helps us to understand the acidic nature of AlCl3? Write a chemical reaction between AlCl3

AlCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, bond angle | Molecular geometry, Molecular, Molecular shapes
When BCl3 is treated with water, it hydrolyses and forms [B[OH]4]^– only whereas AlCl3 in acidified aqueous solution forms [Al (H2O)6]^3+ ion. - Sarthaks eConnect | Largest Online Education Community

Mention the hybridization of the central atom in the given molecule. Molecular AlCl3 | Homework.Study.com

inorganic chemistry - Explanation of bond angles in the aluminium chloride dimer - Chemistry Stack Exchange
6. Which one of the following compounds has the electron pair geometry as the trigonal bipyramid with three equatorial positions occupied by lone pairs of electrons? A) [AlCl3] B) XeF2 C) [
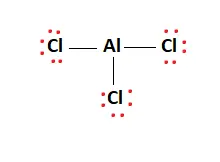
AlCl3 Lewis Structure, Molecular Structure, Hybridization, Bond Angle, and Shape - Geometry of Molecules